CCHF’s Legislative and Policy Specialist, Natasha Chernyavsky, Testimony on HF 1520 (Hemmingsen-Jaeger) – Consent for Direct-to-Consumer Genetic Testing
Testimony on HF 1520 (Hemmingsen-Jaeger) – Consent for Direct-to-Consumer Genetic Testing
Testifier: Natasha Chernyavsky, legislative and policy specialist MN House Commerce Committee, Monday February 20, 2023
Thank you, Mister Chair, and members of the committee.
My name is Natasha Chernyavsky, I am the legislative and policy specialist of Citizens’ Council for Health Freedom.
CCHF has long supported genetic privacy and, although we appreciate the sentiment and goals of HF 1520, as we’ve expressed to Representative Hemmingsen-Jaeger, we are concerned that the bill does not sufficiently protect privacy and individual rights and gives a false sense of security to consumers.
We have testified on this bill on the Senate side and are thankful for the amendments that were incorporated there and here thus far. However, we are still deeply concerned by line 2.25 which states that “genetic data does not include deidentified data.” This bill’s intent is to give consumer’s protection over their genetic data, however under line 2.25 the companies will only be obligated to protect a subset of a consumer’s genetic privacy – anything that is deidentified may be shared without consumer consent. Even though it’s still the person’s blood or the
person’s spit, it will no longer be deemed genetic data, and those specimens and any related data will be exempt from the protective provisions of this bill, including 4.1 to 4.5. This is even more concerning when we realize that, as noted by 23&Me, if the un-pooled individual data is breached, a person could potentially be identified by their deidentified DNA and data by simply using a public database.
Our second concern is on line 3.20 and 3.21, which states that Minnesota policy regarding consent for research is to be defined by the federal Common Rule for federally-funded research. Thus, the bill would transfer authority to federal officials to determine the definition and even the need for obtaining consent for data sharing. The Common Rule provides four reasons that consent requirements can be waived, including whenever an IRB decides there’s “minimum risk.” That should be an individual decision, not a corporate IRB decision. Minnesota should not cede authority to unelected federal officials who can change consent provisions with every new administration or in line with industry lobbying. We’ve never ceded our state authority to determine consent requirements, yet today plenty of federally-funded research takes place every day. We request that language be deleted.
Overall, this bill appears to be less about privacy and more about providing companies with the right to use genetic
data as they wish without consent. Deidentified or not, it’s still genetic data, it’s still DNA, but under this bill it
won’t be identified or protected as such. To truly protect consumers, line 2.25 should also be deleted. Consumers have the right to protect their DNA, regardless of whether it is deidentified or not.
Finally, if this committee intends to pass this bill, we have two more suggestions to strengthen consumer protections — in addition to our request to delete line 2.25 and to delete the reference to CFR 45, part 46.
First, require informed written consent specific to deidentification of the specimen. The consumer would be informed that they lose all rights to where it goes and what happens to it once it’s deidentified, and they’d have a choice in whether it is deidentified. Second, on line 4.4 give consumers access to an accounting of disclosures so they know how often and with whom it was shared.
Thank you for your time and attention to these concerns.



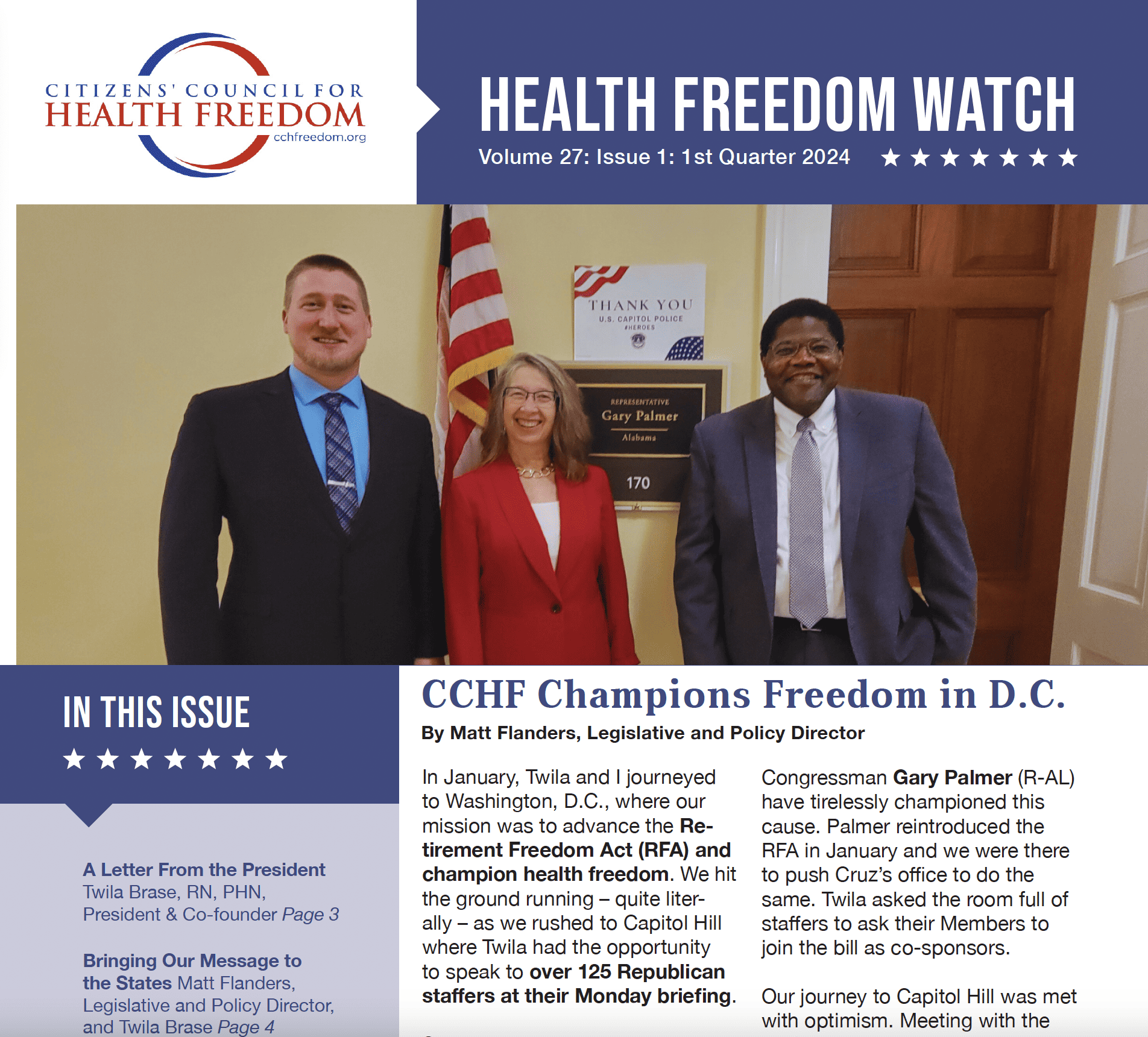
Saving Grace, Good News, and Medicare’s 59th Anniversary