Publications
When it comes to fighting for health freedom, information is key and knowledge is power. You can find CCHF’s latest content – as well as our archived content – here on our website. From original papers and articles to issues of our quarterly Health Freedom Watch, this is where we add published content to keep you informed.

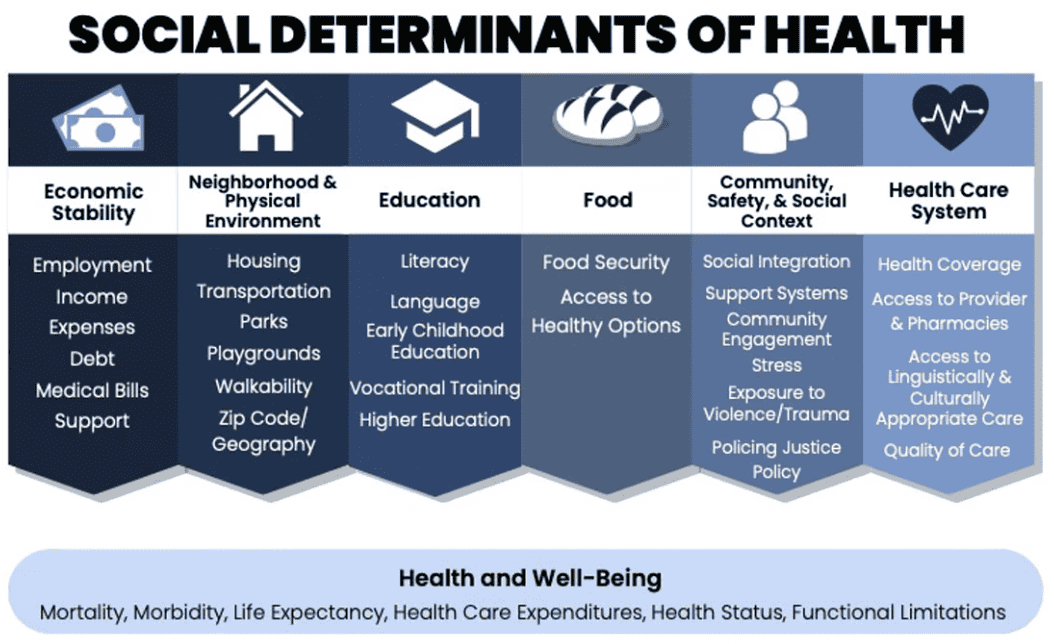
Key Initiatives
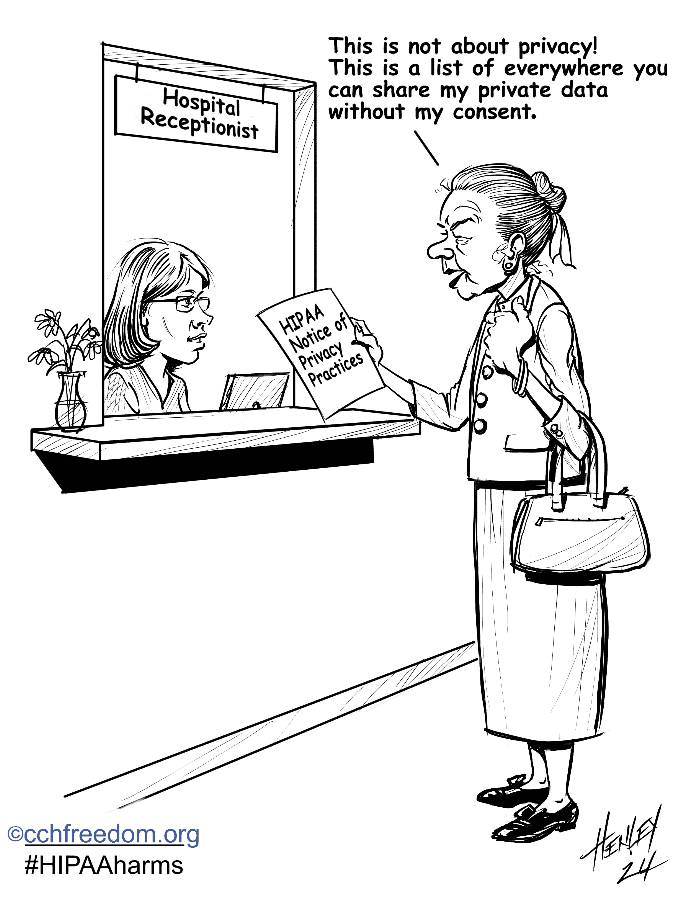
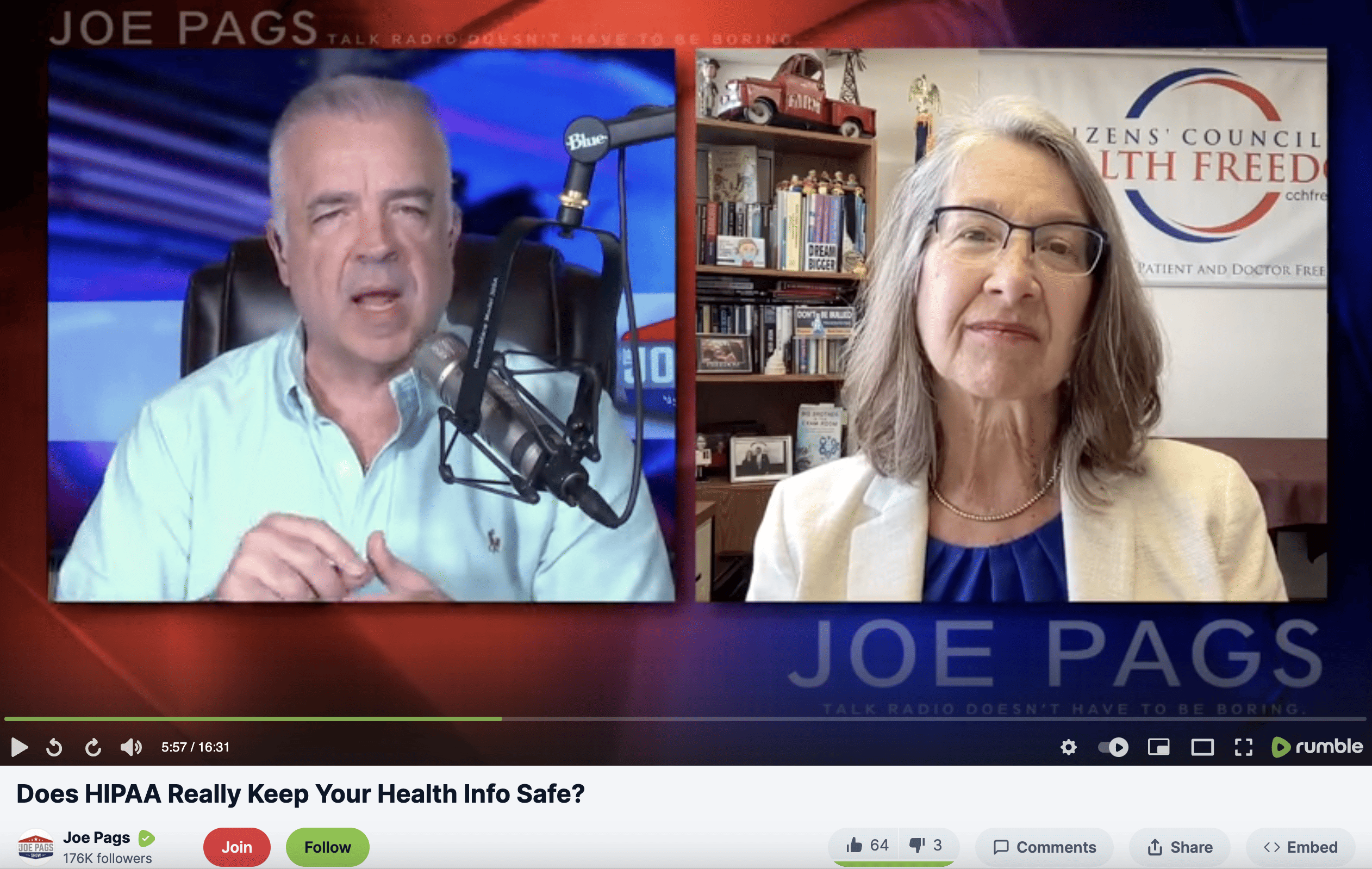
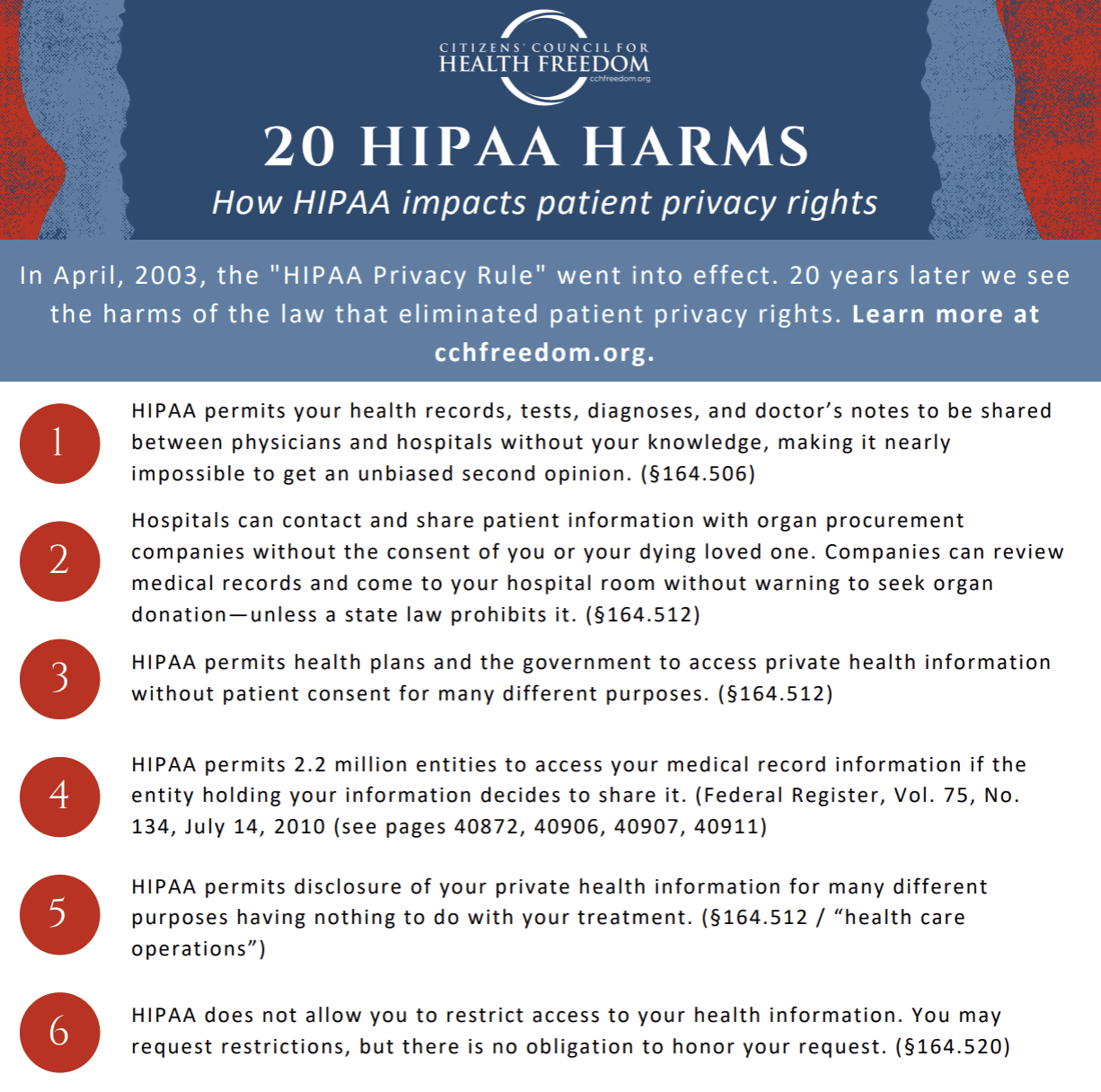
HIPAA: The Deliberate Deception
Working to repeal the permissive data-sharing law that does not protect your patient privacy rights.

REAL ID - Don't Comply
Unconstitutional National ID card paving the way to a possible China-like Digital Social Credit System of biometric control.

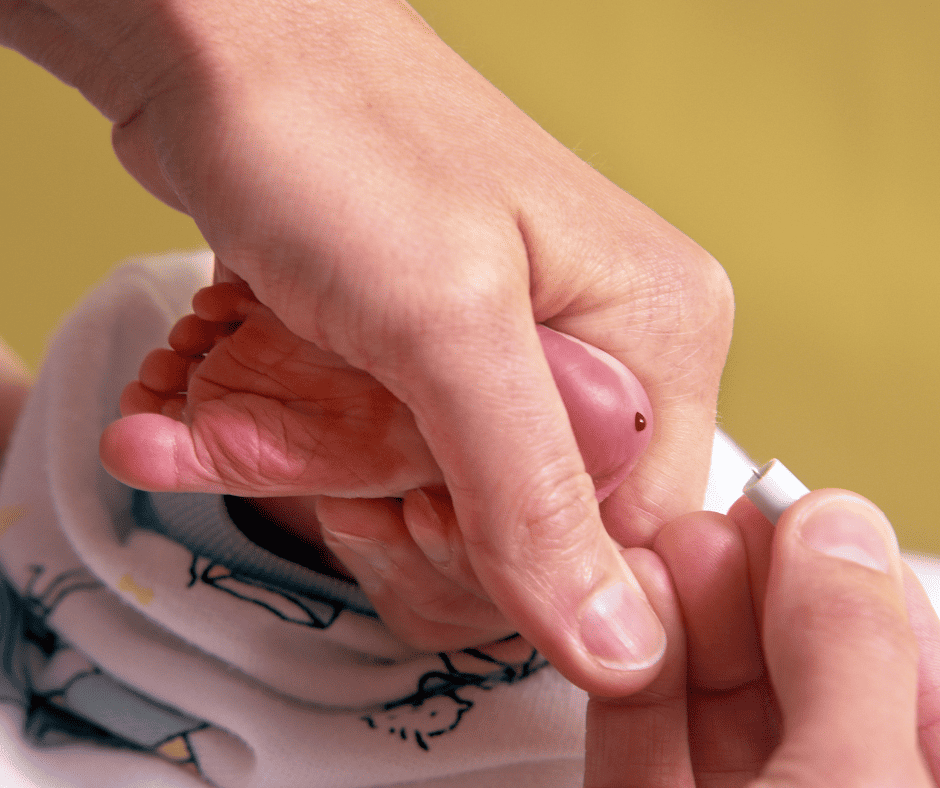
Baby DNA Warehousing
Stopping government storage of newborn DNA without parental consent after
newborn screening.

Find an Independent Doctor Who Works for You
Check out our online nationwide network of cash-based, direct-pay, independent medical practices.

Medicare Opt-Out
Giving seniors the right to opt out of Medicare without losing access to Social Security Retirement Benefits.

Exam Room Access for Parents
Fighting to give parents the right to stay in the exam room to protect their children from intrusive questioning.
Subscribe to the Newsletter
Enter your email to begin receiving monthly newsletter emails.



















































































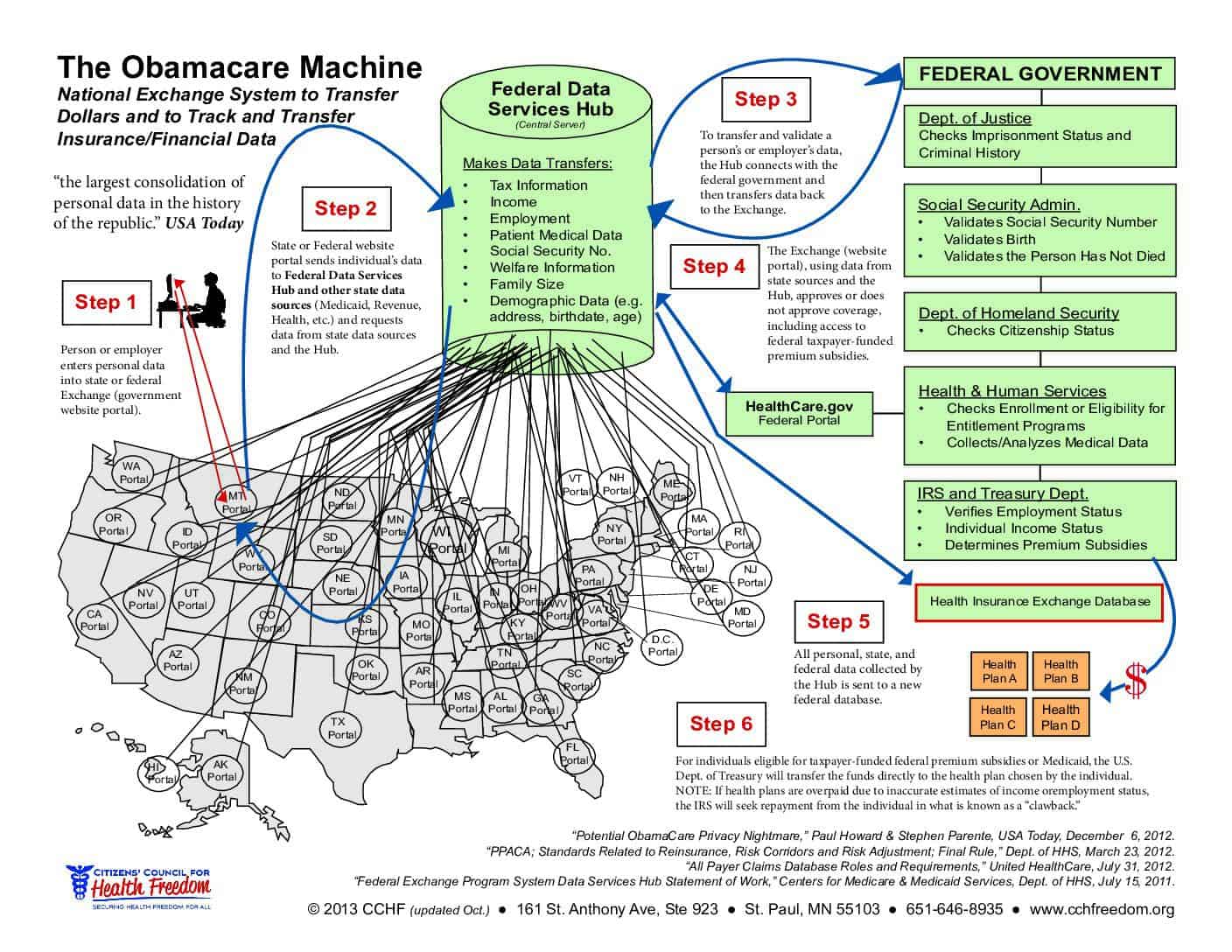
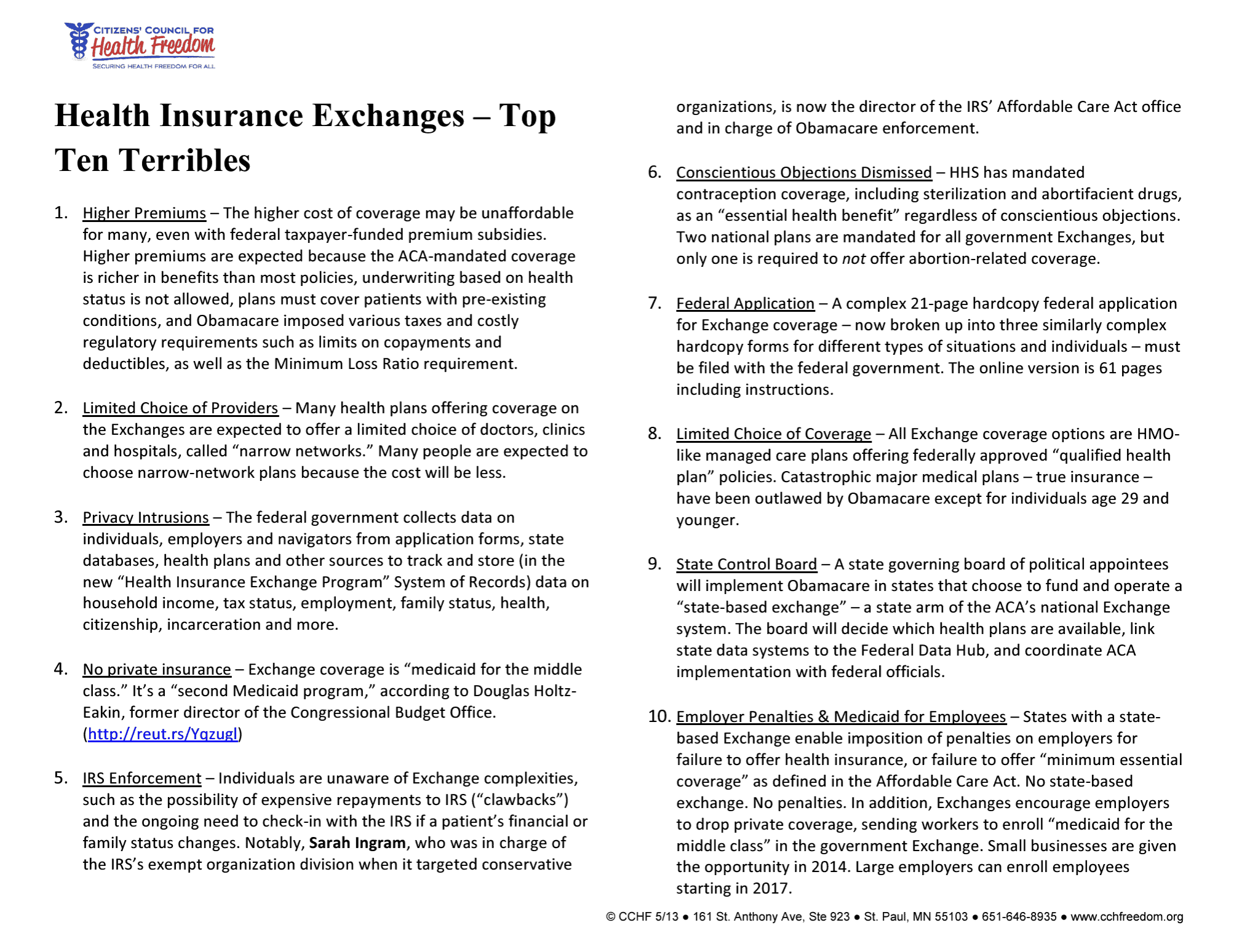







Make an Educated Choice